SAKK 35/10 Lymphoma Trial
Six-month Rituximab-Lenalidomide regimen in advanced untreated Follicular Lymphoma
The Swiss Group for Clinical Cancer Research (SAKK) and the Nordic Lymphoma Group (NLG) conducted the SAKK 35/10 randomized phase-2 trial (NCT0137605) to compare the effectiveness of rituximab (R) alone versus R combined with lenalidomide (L) as the initial treatment for symptomatic follicular lymphoma (FL). The primary endpoint analysis demonstrated higher complete remission rates with the combination therapy.
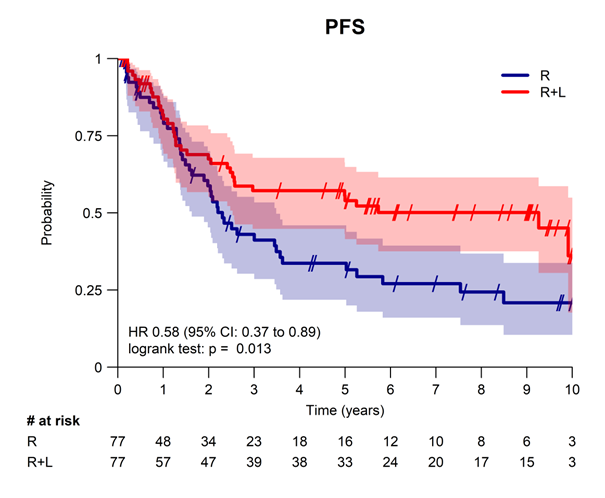
A long-term analysis of time-to-event endpoints with a median follow-up of approximately 10 years shows sustained and significant improvements in the R+L arm in terms of response duration, progression free survival and time to next treatment. Over 60% of RL responders remained in first CR (Complete Response) at 10 years. Overall survival was similar in both arms (77% vs. 78% at 10 years). Notably, the adopted RL schedule is considerably shorter than the standard R-square (R2) regimen (18 vs. 120 weeks). This may provide a promising and safe alternative when treatment is required but durable immunosuppression is undesirable, particularly for frail and elderly patients.